CARBOSILANE DENDRIMERS AND NOVEL DENDRITIC ARCHITECTURES
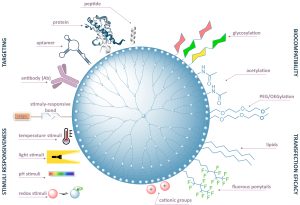
In our research group we are developing new concepts and the corresponding synthetic methods for the preparation of materials which show desirable biological activity, provide for bio-medicinal diagnostics or, as in the case of theranostics, combine both functions. Multivalency is a common principle to increase the affinity and specificity of ligand–receptor interactions in nature and results in a cooperative, over‐additive enhancement of binding affinity.
Our research interest focuses on:
• carbosilane dendrimers
We develop synthetic methods to prepare multivalent carbosilane structures (dendrimers, dendrons, amfifiles, etc.) It has been proved that phosphonium terminated carbosilane dendrimers developed in our laboratories form complexes with therapeutic sequences of nucleic acids (dendriplexes); and, compared to commonly used ammonium analogs, they have lower cytotoxicity and higher transfection efficacy. A modification of a phosphonium dendron with a non-polar carbohydrate chain in the focal point leads to amphiphilic molecules, which form various supramolecular assemblies (micelles, liposomes, etc.). Therefore, such compounds open new opportunities in gene therapies and biomedical applications. Furthermore, applications of multivalent and branched compounds offer solutions in the field of catalysis. We develop highly efficient catalytic systems, optimize reaction conditions and subsequent processes to maximize a general sustainability of the catalytic reactions.
• branched and dendritic modules
• glycodendrimers
Synthetic multivalent carbohydrates, especially well defined glycodendrimers, represent efficient tool for study of glycobiological processes in living systems, which are important in various processes. Currently, we are the only groups developing carbosilane glucodendrimers (glyco-CS-DDMs) as class of well-defined functional bionanomaterials.